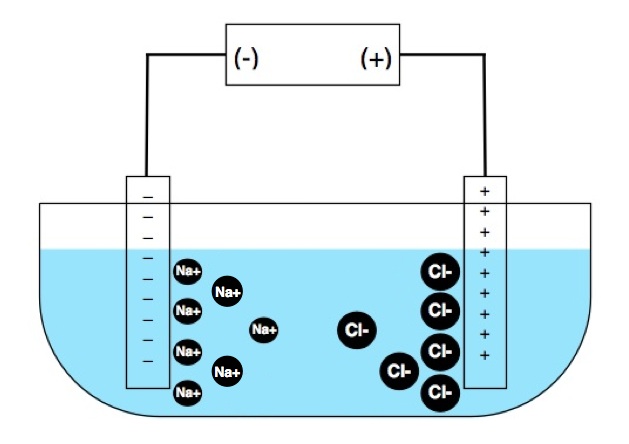
Ions traveling toward the electrodes will quickly form dense clouds near the electrode surfaces. They cannot physically cross the solid/liquid barrier, and the voltage is usually too low for significant electrolysis to occur (forming elemental sodium and chlorine gas for our example.) But the cloud of negative ions will present a rising counter emf at the positive electrode, and vice-versa. The result is that current flow rapidly and exponentially drops to zero, invalidating the measurement. To overcome this, an alternating polarity voltage is applied. This can be a sine wave, square wave, trapezoid, or any number of forms. The purpose of using AC is to reverse the motion of the ions before they build up near the electrode surfaces in any quantity.
This simplified description does not address lesser but still serious problems with reduced but not eliminated polarization that are overcome with various electronic tricks. Frequency manipulation, current sensing windows, peak detectors and other devices are used to make sure that the current monitored is representative of the ion population and not polarization.

No comments yet. You should be kind and add one!
Our apologies, you must be logged in to post a comment.